Tissue Hypoxia Definition

Tissue hypoxia is a condition in which tissue cells experience inadequate oxygen utilization due to a number of causes such as decreased partial pressure of oxygen (PO2) in a given tissue.
Endotoxins associated with sepsis or other critical conditions may also inhibit cellular metabolism and decrease oxygen consumption. Cellular utilization of oxygen may be inhibited by some metabolic poisons, such as cyanide. Learn more about Aerobic Cellular Respiration...
Releasing various toxins and/or mediators one organ affected by tissue hypoxia can lead indirectly to a dysfunction or even failure of other organs. Measurement of changes in oxygen consumption in response to changes in oxygen delivery is one of the methods used to determine existence of tissue hypoxia.
Some regulatory mechanisms involved in the stimulation and regulation of angiogenesis are comparable to those implicated in collagen synthesis and deposition. Oxygen stimulates macrophages to produce angiogenic substances (like vascular endothelial growth factor [VEGF]) that attract and stimulate endothelial cells.264 Therefore, hypoxia weakens the neovascularization process.
A decrease in venous oxygen saturation can be caused by either decrease in oxygen delivery, increase of oxygen demand or both. Metabolic acidosis developed in hypoxic tissues is one of the common abnormalities associated with tissue hypoxia.
Injuries that damage the microvasculature attract inflammatory cells that consume large amounts of oxygen and concentrate potentially damaging products at the hypoxic tissues of wound site. This creates a low-oxygen environment with low pH, high lactate, increased oxidant production and poor local perfusion.261 The macrophages respond to this environment by releasing growth factors that induce angiogenesis, multiplication of fibroblasts and collagen synthesis. Therefore, acute wound hypoxia is, to a certain extent, necessary for leukocyte adherence, neovascularization, collagen formation and bone formation.262
However, oxygen availability becomes essential in 2 steps of collagen biosynthesis proline and lysine are incorporated into growing peptides and hydroxylated when they enter the endoplasmic reticulum. When the PO2 is 20 mm Hg, this process evolves at half the normal rate; when the PO2 is 200 mm Hg, this process evolves at 90% of the optimal rate. Moreover, cell multiplication also requires oxygen. An oxygen environment of 40 mm Hg is needed to ensure fibroblast activity.263 Therefore, chronic wound hypoxia weakens the collagen synthesis process.262, 264
Analyzing the underlying causes and clinical manifestations of tissue hypoxia in each individual case is crucial, because different conditions may promote tissue hypoxia in different ways. Maintaining an adequate blood supply to the oxygen-sensitive tissues should be among primary corrective measures when treating tissue hypoxia.
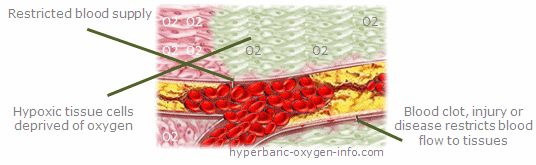
There is a tremendous need for treatments that will reduce the human and economic burden and loss associated with diabetic foot ulcers and lower extremity ulcerations. As tissue hypoxia is one of the pathophysiological characteristics of diabetic ulcers, Hyperbaric Oxygen Therapy (HBOT) has been considered as a therapeutic strategy to reduce tissue hypoxia and enhance wound healing.
If you prefer to learn more about hypoxia by reading a textbook, we recommend a selection of books. Providing a detail overview of hypoxia, hypoxia symptoms and types, the recommended books address the distinctive problems that hypoxia presents to vulnerable organs such as the kidney, liver, heart and brain.
Tissue Hypoxia In Wound Healing Failure
©2008 Undersea and Hyperbaric Medical Society, Inc. The book can be purchased at UHMS web site.
By Robert A. Warriner III, M.D., FACA, FCCP, CWS and Harriet W. Hopf, M.D.
Normal wound healing proceeds through an orderly sequence of steps involving control of contamination and infection, resolution of inflammation, regeneration of the connective tissue matrix, angiogenesis, and resurfacing. Several of these steps are critically dependent upon adequate perfusion and oxygen availability. The end result of this process is sustained restoration of anatomical continuity and functional integrity.
Problem or chronic wounds are wounds that have failed to proceed through this orderly sequence of events and have failed to establish a sustained anatomic and functional result. This failure of wound healing is usually the result of one or more local wound or systemic host factors inhibiting the normal tissue response to injury. These factors include persistent infection, malperfusion and hypoxia, cellular failure, and unrelieved pressure or recurrent trauma.
The hypoxic nature of all wounds has been demonstrated, and the hypoxia, when pathologically increased, has correlated with impaired wound healing and increased rates of wound infection. Local oxygen tensions in the vicinity of the wound are approximately half the values observed in normal, non-wounded tissue. The rate at which normal wounds heal has been shown to be oxygen dependent.
Fibroblast replication, collagen deposition, angiogenesis, resistance to infection, and intracellular leukocyte bacterial killing are oxygen sensitive responses essential to normal wound healing. However, if the periwound tissue is normally perfused, steep oxygen gradients from the periphery to the hypoxic wound center support a normal wound healing response.
In the hypoxic wound, hyperbaric oxygen therapy acutely corrects the pathophysiology related to oxygen deficiency and impaired wound healing. A key factor in hyperbaric oxygen therapys enhancement of the hypoxic wound environment is its ability to establish adequate oxygen availability within the vascularized connective tissue compartment that surrounds the wound.
Proper oxygenation of the vascularized connective tissue compartment is crucial to the efficient initiation of the wound repair process and becomes an important rate-limiting factor for the cellular functions associated with several aspects of wound healing. Neutrophils, fibroblasts, macrophages, and osteoclasts are all dependent upon an environment in which oxygen is not deficient in order to carry out their specific inflammatory or repair functions. Two groups of induced responses occur:
- Improved leukocyte function of bacterial killing, antibiotic potentiation, and enhanced collagen synthesis occur during periods of elevated tissue PO2.
- Suppression of bacterial toxin synthesis, blunting of systemic inflammatory responses, and prevention of leukocyte activation and adhesion following ischemic reperfusion are effects that may persist even after completion of hyperbaric oxygen treatment.
In addition, vascular endothelial growth factor (VEGF) release is stimulated and platelet derived growth factor (PDGF) receptor appearance is also induced. The net result of serial hyperbaric oxygen exposures is improved local host immune response, clearance of infection, enhanced tissue growth and angiogenesis with progressive improvement in local tissue oxygenation, and epithelialization of hypoxic wounds.
Local wound hypoxia plays a pivotal role in diabetic wound healing failure and limb loss as evidence by the report by Pecoraro that when periwound PtcO2 values were below 20 mmHg they were associated with a 39 fold increased risk of primary healing failure. While aggressive distal lower extremity bypass grafting and lower extremity angioplasty have contributed to increased wound healing and limb salvage rates, technical grafting success does not necessarily equate with limb salvage. Hyperbaric oxygen treatment offers an intriguing opportunity to maximize oxygen delivery in the setting of minimal or insufficiently corrected blood flow.121
Read more about Hypoxia, Hypoxic Hypoxia, Histotoxic Hypoxia, Cerebral Hypoxia, Chronic Hypoxia, Altitude Sickness.